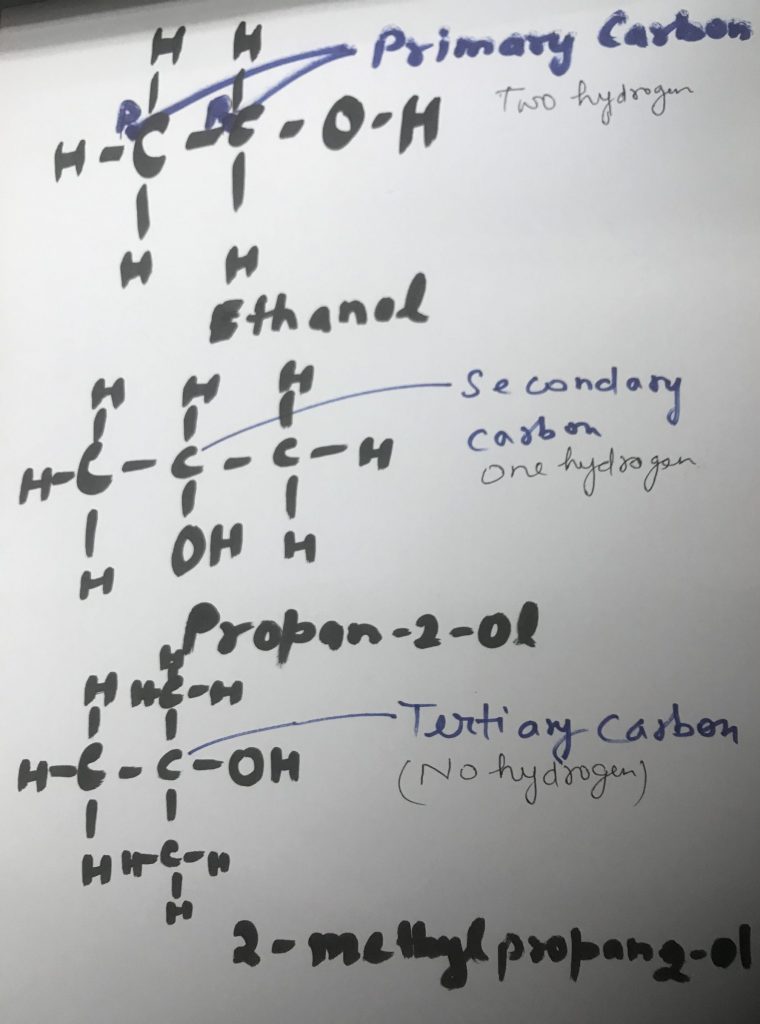

Primarycarbon (1ocarbon): A carbon directly bondedto just one other carbon group.
- A primary (1º) carbon is a saturated carbon in an organic species bonded to only one carbon atom. Eg: see also secondary carbon, tertiary carbon, quaternary carbon.
- For reactivity using an Sn2 mechanism, primary secondary tertiary carbon centers. On the other hand, Sn1 reactions are unimolecular in rate of reaction and have a step-wise mechanism. This process first involves bond cleavage by the LG to generate a carbocation intermediate.
How can a functional group be added to a primary carbon?
Primary Carbon Sn2
1 Answer
There are many answers to this question, but I'll give you a few quick ones.
Primary Carbon Organic Chemistry
Assuming your primary carbon is an alkene you will want to do an anti-markovnikov alkene addition reaction.
The 2 common ones you likely learned are adding an OH or halogen
Adding and OH
The hydroboration oxidation reaction will add an OH to the primary carbon. If you want an alcohol, stop here
If you want an aldehyde react this with PCC
If you want a carboxylic acid, react this with a stronger oxidizing agent.
If you want a carboxylic acid derivative - start by turning it into a carboxylic acid, then react with SOCl2 turning it into an acyl chloride.
Now you have a reactive group that can be turned into an ester, amide, anhydride and more
Adding a Halogen
Another route is to add a halogen under radical conditions such as H-Br in the presence of peroxide.
This adds anti-markovnikov to the primary carbon.

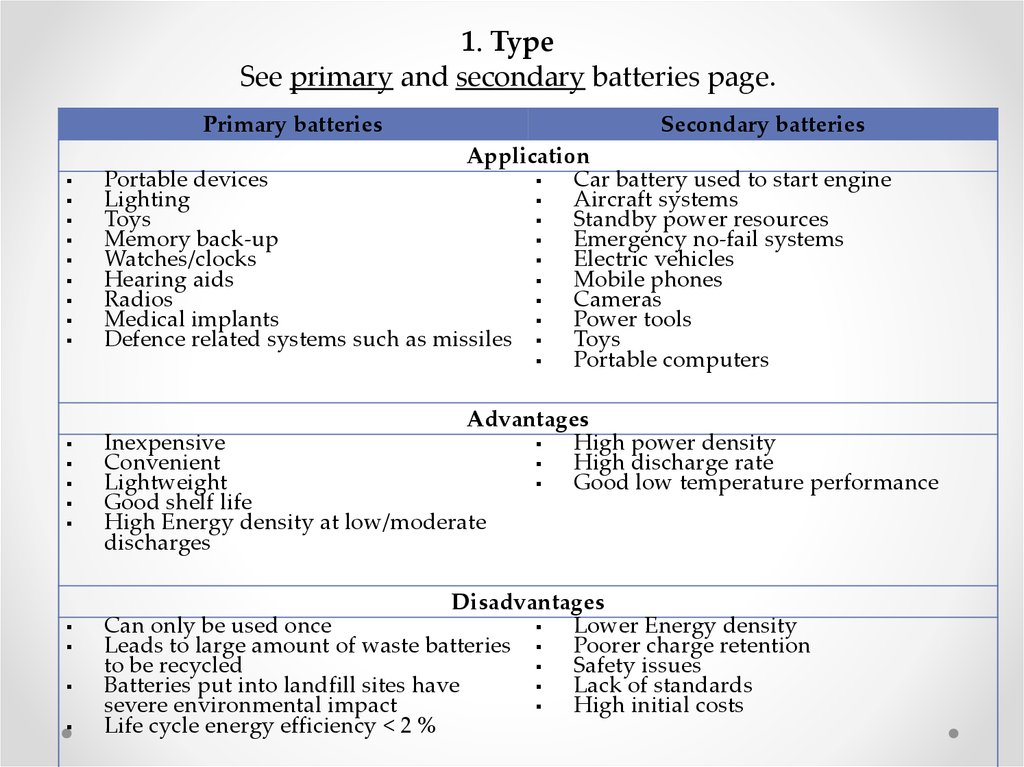

Now that you have a primary bromide, you can do an SN2 reaction to replace the Br with a wide variety of functional groups
I cover many examples of SN2 reactions on my website here:
http://leah4sci.com/nucleophilic-substitution-and-beta-elimination-sn1-sn2-e1-e2-reactions/
Related topic
Primary Carbon Footprint
Related questions
The slow cycle returns carbon to the atmosphere through volcanoes. Earth’s land and ocean surfaces sit on several moving crustal plates. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure. The heated rock recombines into silicate minerals, releasing carbon dioxide.
When volcanoes erupt, they vent the gas to the atmosphere and cover the land with fresh silicate rock to begin the cycle again. At present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. For comparison, humans emit about 30 billion tons of carbon dioxide per year—100–300 times more than volcanoes—by burning fossil fuels.
Primary Carbon Substrate
Chemistry regulates this dance between ocean, land, and atmosphere. If carbon dioxide rises in the atmosphere because of an increase in volcanic activity, for example, temperatures rise, leading to more rain, which dissolves more rock, creating more ions that will eventually deposit more carbon on the ocean floor. It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering.
